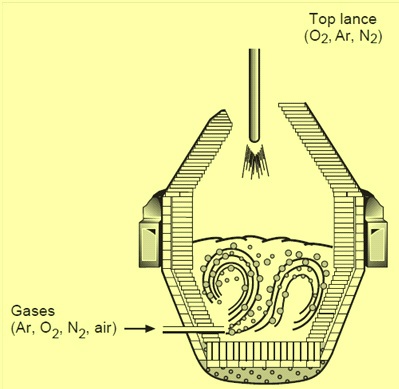
Argon Oxygen Decarburization Process
Argon oxygen decarburization (AOD) is a process primarily used in production of stainless steel and other high grade alloys such as silicon steels, tool steels, nickel-base alloys and cobalt-base alloys with oxidizable elements such as chromium and aluminum. AOD was invented in 1954 by the Lindé division of The Union Carbide Corporation, which became Praxair in 1992. An AOD converter is shown in Fig 1.
Fig 1 AOD converter
Today, over 75 % of the world’s stainless steel is made using the AOD process. The process is very popular because it combines higher metallic yields with lower material costs. It provides an economical way to produce stainless steel with a minimum loss of precious elements. It is part of a duplex process in which scrap or virgin raw materials are first melted in an electric arc furnace (EAF) or induction furnace (IF). The molten metal is then decarburized and refined in an AOD converter to less than 0.05 % carbon. The key feature in the AOD converter is that oxygen for decarburization is mixed with inert gas such as argon or nitrogen and injected through submerged tuyeres. This argon dilution of oxygen minimizes unwanted oxidation of precious elements contained in specialty steels, such as chromium. Other benefits of AOD process include pinpoint accuracy in chemistry control down to 0.01 % carbon and lower, rapid desulfurization to less than 0.001 %, and lead removal to less than 0.001 %. The end result is a cleaner metal coupled with increased productivity.
AOD process uses dilution technique for the decarburization of steel bath. The injection of inert gas (argon or nitrogen) lowers the partial pressure of CO in the bath, thus allowing higher chromium content to be in equilibrium with lower carbon contents. The amount of stirring energy from the gas blown through the subsurface tuyeres and the formation of the carbon monoxide deep within the metal bath results in the converter processes being among the most intensely stirred metallurgical reactors. The intimate gas – metal contact and excellent slag – metal mixing facilitate refining reactions.
AOD process refining has three major steps. These are (i) decarburization, (ii) reduction, and (iii) final chemistry and temperature trimming.
The input of the AOD process is the output of the EAF or IF process. The liquid steel, which contains most of the chromium and nickel needed to meet the final heat composition, is tapped at a temperature of 1500 to 1600 deg C from the EAF or the IF into a transfer ladle. The liquid metal is transferred from transfer ladle to AOD converter. The AOD converter can be rotated downwards so that the side mounted tuyeres are above the bath level during charging of the liquid steel.
After the transfer of liquid steel containing iron, chromium, carbon and nickel from EAF or IF to the AOD converter, high carbon ferro chrome is added and the blow is started with the blowing of inert gas (argon, nitrogen) and oxygen mixture. In the initial stage, oxygen to argon in the ratio ranging from 5:1 to 3:1 is blown through the side tuyeres. The ratio is lowered with the progress of the decarburization. Since the blowing is done along with argon it is possible to carry out the decarburization at a lower temperature. When carbon reduces to 30 % of the original value the ratio of oxygen to argon is changed to 2:1. The major benefit associated with the dilution process comes into play when the oxygen to inert gas ratio is 1:1. Oxidation of carbon continues, but oxidation of chromium is limited. This is due to the very low oxygen potential of the gas mixture, which minimizes chromium oxidation. The blow is continued to attain 0.09 % to 0.012 % C.
Process gases are injected through submerged tuyeres that are installed in the side wall or bottom of the converter. Side wall injection usually imparts maximum stirring energy to the bath for greatest efficiency of mixing. Bottom injection usually improves wear characteristics in the barrel section of the converter. The number and relative positioning of tuyeres is determined in part by converter size, range of heat sizes, process gas flow rates and types of alloys refined.
The gas control system supplies the process gases at nominal rates of 1.0–3.0 N cum/min/ton. The system accurately controls the flow rates and monitors the amount of gas injected into the bath to enable the operator to control the process and measure the total oxygen injected.
Decarburization occurs when dissolved carbon reduces the chromium and iron oxides that form. The decarburization reactions are as follow.
3O? (g) + 4Cr = 2Cr?O?
Cr?O? + 3C = 2 Cr + 3CO (g)
Decarburization occurs on the surface of rising bubbles that form from either the inert gas that is injected or on the surface of chromium oxide particles that are being reduced and generating carbon mono oxide (CO).
During decarburization, additions are made for obtaining the proper final chemical composition. These additions usually consist of desired amounts of high carbon ferrochromium, stainless steel scrap, carbon steel scrap, nickel, iron, high carbon ferromanganese, and molybdenum oxide. These additions also serve to reduce the bath temperature as carbon and chromium oxidations are exothermic. In general, the bath temperature is controlled to less than 1720 deg C. Total weight of alloy addition is in the range of 5 % to 30 % of the tap weight. During the final stage of blowing, the ratio of oxygen to argon is changed to 1:3 to 1:2 for bringing carbon to the desired value which can be less than 0.03 %.
The next step is the reduction step, in which the reduction additions are charged and stirred with an inert gas for a desired time. The reduction mix consists of silicon alloys, such as ferrosilicon or chromium-silicon, and/or aluminum, which are added for the reduction of metallic oxides from the slag and fluxing agents such as lime, dolomitic lime, and fluorspar. The bath is then stirred with inert gas, typically for around five to eight minutes.
Cr?O? + 2Si = 3Cr + 2 SiO?
Additional silicon addition is needed if requirement of silicon is there to meet the silicon specification of some of the stainless steels.
Careful manipulation of slag, as it precipitates in the reaction, is important. Any chromium oxide not reduced by carbon ends up in the slag, which can form a complex spinel. The effectiveness of reduction step is dependent on many factors including slag basicity and composition, temperature, mixing conditions in the converter and solid addition dissolution kinetics.
Lime and/or dolomitic lime are usually added just before the oxygen blow to flux the transfer slag and silicon in the metal. During the oxygen blow, silicon is oxidized before carbon. Lime and dolomitic lime are sometimes added before the end of the blow to cool the bath and to reduce the volume of reduction additions. Slag fluxing additions, such as lime, dolomitic lime and spar, are typically in the range of 3 % to 7 % of total bath weight.
The formation of a high basicity slag and the reduction of oxygen potential in the metal bath are good conditions for sulfur removal. For example, with starting sulphur of 0.03 %, a reduction treatment of 2-3 kg aluminum/ton, 2 – 3 kg spar/ton, final slag basicity of about 1.7, and temperature of 1700 deg C, finish sulfur contents of 0.003 – 0.005 % can be obtained.
The length of the blow period is determined by the starting carbon and silicon levels of the hot metal charged to the AOD converter. Decarburization time ranges from 20 to 35 minutes in modern converters (start from 1.5 % to 2.5 % and aim carbon 0.04 %). Usually, the converter is turned down to a horizontal position and a sample of the liquid steel is taken for analyses at a carbon level of about 0.1 %.
Sulfur removal is a slag – metal reaction that occurs during the reduction phase of the process. Phosphorus, which requires oxidizing conditions, cannot be removed in the converter processing.
Nitrogen control is a gas – metal reaction. Depending on final nitrogen specification for the stainless steel grade, the inert gas during the initial stages of decarburization can be nitrogen. After a certain carbon level is achieved, the nitrogen gas is replaced by argon. Such an approach is usually practiced by steelmakers to reduce argon usage and costs and still achieve a desired nitrogen specification. After the change from nitrogen to argon, nitrogen is removed from the bath both by evolved carbon monoxide and argon. Volatile elements with high vapour pressures, such as lead, zinc, and bismuth, are removed during the decarburization period.
The formation of high basic slag and the reduction of oxygen potential in the liquid steel bath are good conditions for sulphur removal. These are achieved by having a high lime concentration in the slag and a low oxygen activity in the metal bath. The transfer of sulphur to slag takes place as per the following reaction.
S(bath) + CaO(slag) = CaS (slag) + O(bath)
Additions of lime are made to dilute the sulphur in the liquid steel bath. Also, aluminum or silicon may be added to remove oxygen. For example, with a start sulphur of 0.03 %, a reduction treatment of 2 to 3 kg aluminum/ton, 2 to 3 kg spar/ton, final slag basicity of about 1.7 and a temperature of 1700 deg C would bring down the sulphur content to 0.003– 0.005 %. If the grade to be produced requires an extra low sulphur level, the bath is deslagged after the reduction step and another basic slag is added. The liquid steel and the fluxes are then mixed to complete the desulfurization reaction. In modern practices a sulphur level of 0.001 % or less is easily achieved with this double slag practice. Other trimming alloy additions might be added at the end of the step. After sulphur levels have been achieved the slag is removed from the AOD vessel and the metal bath is ready for tapping.
Ideally at this stage of the process, the chemistry of the liquid steel should meet the final specifications so that the heat can be tapped. If necessary, additional raw materials may be charged for small chemistry adjustments before tapping. After tapping, the ladle is often stirred for composition homogenization and temperature uniformity along with flotation of inclusions. This is done in a ladle equipped with stirring facilities with or without the use of a ladle furnace. After the ladle treatment, the steel is ready to be cast. In the early days of the AOD process, the converter was tilted for raw material additions as well as for taking samples and for measurement of temperature using immersion thermocouples. The desire to increase the productivity has led to continuous charging of raw materials during the blow period as well as reduction period. Modern instrumentation has been developed which can take melt samples as well as steel temperatures using a specially designed sub lance with the converter in the upright position.
AOD converter
AOD converter is a pear shaped vessel usually lined with basic refractory lining. It has a removable, conical cover in place. The important feature of an AOD converter is that it is normally side blown. In case of those steel grades which can tolerate nitrogen, a mixture of oxygen and nitrogen can also be blown. As molten stainless steels do not generate foam, and most stainless steel refining processes are side or bottom-blown, the dimensions of a stainless refining converter are smaller than a comparable BOF (basic oxygen furnace) converter. Typical internal volumes of AOD converters are in the range 0.4 – 0.8 cum /metric ton bath weight.
For converters that tap into a ladle held by a crane, a sliced cone top section is often used. The slice portion allows the crane to come close to the converter mouth. Converters that tap into a ladle car usually have a BOF type concentric cone top section.
A high production shop typically has three interchangeable converters for 100 % availability of the process. At any given time, one of the converters is in the tiltable trunnion ring refining steel, a second newly lined converter is at a preheating station, and the third converter is at a reline station. The converter in the trunnion ring typically can be replaced with a preheated converter in less than an hour.
The AOD converter has tuyeres mounted in the sidewall or in the bottom. These tuyeres typically consist of a copper tube with a stainless steel outer tube. An annulus is formed between the copper and stainless tubes. Cooling gases blown through the outer annulus (shroud) form a metal or oxide accretion (called a mushroom) at the tuyere tip. This accretion protects the tuyere and surrounding refractory. Process gases of oxygen/inert mixtures blow through the inner annulus. Special designs exist for normalizing the flow in the annular gap. Tuyere size and number depend on specific process parameters. There are usually between two and nine tuyeres in an AOD vessel.
Sidewall mounted tuyeres are submerged while processing. When the vessel is rotated, the tuyeres are above the bath. At this point, the process gases can be shut off and a small cooling flow protects the tuyeres
Bottom blown converters have a variety of tuyere configurations depending on flow rates required. There are usually two to four tuyeres in the bottom.
A major modification of the AOD process involves the use of top blowing lance in addition to the side blowing tuyeres. The lance can be used to inject oxygen at desired blow rates to increase decarburization and/or post combustion. The top lance can also be designed for blowing mixed gases such as inert gas – oxygen mixtures. The installation of a lance and introduction of oxygen in the early stages of decarburization can reduce the time for a heat. The technology can be used to increase the productivity (tons/hour) of the steel melting shop. Most of the recent converter installations include the use of a top lance for blowing oxygen.
Another modification of the AOD process involves applying vacuum on the converter to reduce the consumption of argon and silicon as well as the process time when making low carbon grades. The modification is known as AOD-VCR.
AOD converter refractories
High temperatures at the tuyere tip and high bath agitation place great demands on the converters refractory. While typical BOF refractory campaigns are months or years long, stainless converter campaigns are several days or weeks long. Refractory costs are a significant fraction of total operating costs.
There are two basic choices of refractory type, magnesite-chromite, and dolomite. The choice of refractory is dependent on the vessel operation pattern, final product specifications, and economics.
Magnesite chromite refractories have high wear resistance but have a higher unit cost than dolomitic refractories. Chromium pickup from the brick is possible. Magnesite chromite bricks are simultaneously acidic and basic and strict slag compositions must be maintained to prevent rapid wear.
Dolomitic refractories are usually less costly than magnesite chromite refractories and chromium pickup is not a factor. Desulphurization to very low levels is generally easier in dolomitic refractories because very basic slags can be used without detrimental effects on the bricks.
Converters are typically zoned by thickness and brick quality to maximize lining life and minimize costs. High wear areas of the converter, usually the tuyere wall, slag line, and transfer pad are zoned thicker and with higher quality refractory than other parts of the converter.